Sodium hydrogen phosphate formula, also named as Sodium phosphate dibasic formula or Disodium phosphate formula is discussed in this article. The molecular or chemical formula of Sodium hydrogen phosphate is Na2HPO4.
In its anhydrous form, it appears as is a white, odourless powder. It is hygroscopic and has a saline taste. In its hydrated form, it appears as a white crystalline solid which is odourless. This inorganic compound is soluble in water but insoluble in alcohol.
It can be obtained by neutralizing phosphoric acid (H3PO4) with sodium hydroxide NaOH. At the industrial scale, it is prepared in a two-step process.
Step 1 – Dicalcium phosphate is treated with sodium bisulfate, to give precipitated calcium sulfate
Step 2 – The obtained monosodium phosphate solution is neutralized partially.
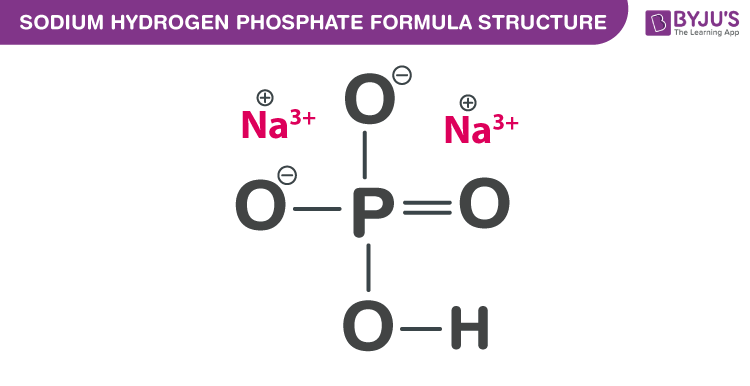
Sodium hydrogen phosphate Formula Structure
Properties Of Sodium hydrogen phosphate Formula
| Chemical formula | Na2HPO4 |
| Molecular weight | 141.96 g/mol (anhydrous) |
| Density | 1.7 g/cm3 |
| Appearance | Crystalline solid |
| Melting point | 250 °C |
To learn more about Sodium hydrogen phosphate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Disodium phosphate for free.
Comments